What does Boyle’s Law state?
- Figure shows an air pillow. When the boy puts his head on the pillow, the air in the pillow is compressed. The pressure of the air increases to support his head.
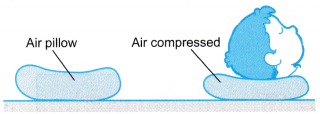
- Boyle’s law gives the relationship between the pressure and the volume of a fixed mass of gas at constant temperature.
- The relationship between the pressure and the volume of a gas can be explained using the kinetic theory of gases.
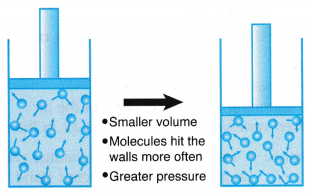 (a) When the volume of a gas is decreased, the number of molecules per unit volume increases.
(a) When the volume of a gas is decreased, the number of molecules per unit volume increases.
(b) The same number of molecules move in a smaller space as shown in Figure.
(c) The molecules collide more frequently with the walls of the container.
(d) The increase in the rate of collision results in an increase in the pressure exerted by the gas. - Boyle’s law states that for a fixed mass of gas, the pressure of the gas is inversely proportional to its volume when the temperature is kept constant.
- The mathematical expression for Boyle’s law:
 that is, PV = constant when the temperature is kept constant.
that is, PV = constant when the temperature is kept constant. - When the pressure changes from P1 to P2 the volume changes from V1 to V2.
 The product of pressure and volume remains constant. Therefore, P1V1 = P2V2.
The product of pressure and volume remains constant. Therefore, P1V1 = P2V2. - The equation for Boyle’s law is:
 when temperature and mass are constant.
when temperature and mass are constant.
P1 = intial pressure P2 = final pressure
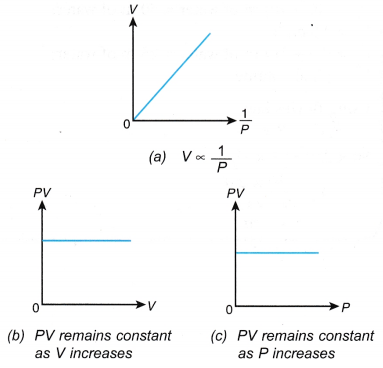
V1 = initial volume V2 = final volume - The relationship between the pressure and the volume can also be expressed with the graphs in Figure.
People also ask
What is the Charles Law?
What is the Pressure Law?
Experiment:
Inference: The pressure on a gas affects its volume.
Aim: To determine the relationship between the pressure and the volume of a fixed mass of gas at constant temperature.
Problem: What is the relationship between the pressure and the volume of a fixed mass of gas at constant temperature?
Hypothesis: The volume of a fixed mass of gas decreases when the pressure is increased.
Variables:
(a) Manipulated variable: Pressure
(b) Responding variable: Volume of the air
(c) Fixed variables: The mass of the air in the syringe and the temperature
Apparatus: A set of Boyle’s law apparatus consisting of a graduated glass syringe, standard weights, Bourdon gauge, rubber tubing, retort stand
Method:
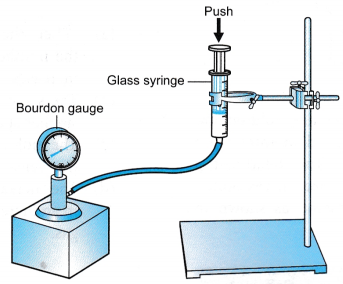
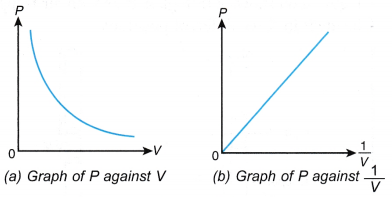
- The apparatus is set up as shown in Figure.
- The piston is pushed down until the Bourdon gauge shows a pressure, P = 110 kPa.
The volume, V, of the air in the syringe is recorded. - Steps 2 and 3 are repeated with pressures, P = 120 kPa, 130 kPa, 140 kPa and 150 kPa.
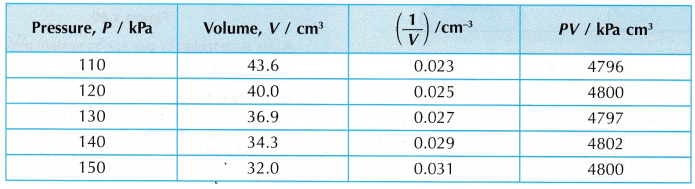
- The values of pressure, P, volume, V, 1/V and PV are tabulated.
- Graphs of P against V and P against 1/V are plotted.
Results:
- Tabulation of results.
- Graphs of P against V and P against 1/V.
Discussion:
- Some grease is applied on the piston so that it can move smoothly into the glass syringe.
- The piston moves slowly into the syringe after a standard weight is added. This ensures that the temperature of the air in the syringe remains constant.
- The graph of P against V shows that the volume of the gas decreases when its pressure is increased.
- The graph of P against 1/V is a straight line passing through the origin. This shows that P ∝ 1/V, that is, the pressure is inversely proportional to the volume.
- The product, PV, is said to be constant within the limits of experimental errors. This also shows that the pressure is inversely proportional to the volume.
Conclusion:
The volume of the air in the glass syringe is inversely proportional to the pressure. The hypothesis is accepted.
Boyle’s Law Example Problems with Solutions
Example 1. The air in a syringe has an initial volume of 12.0 cm3 and pressure of 100 kPa. The nozzle of the syringe is closed and the piston is pushed inwards until the volume of the air becomes 7.5 cm3. What is the pressure of the compressed air in the syringe?
Solution:

Example 2. A deep-sea diver breathed out a bubble of air with a volume of 2.0 cm3 at a depth of 40 m. What will be the volume of the bubble when it is 15 m below the surface of the sea?
[Atmospheric pressure = 10 m of water]
Solution:

Example 3. Figure shows a thin glass tube with air trapped inside it in three different positions.
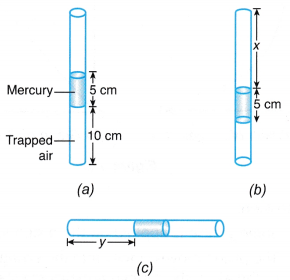
Determine the values of x and y.
[Atmospheric pressure = 76 cm Hg]
Solution:
