Qualitative Analysis of Salts
What is qualitative analysis?
- Qualitative analysis of a salt Analysis is a chemical technique used to identify the ions present in a salt by analysing its physical and chemical properties and hence determine the identity of the salt.
- It determines only the presence or absence of a particular ion in a given salt. This method does not determine how much of a particular ion is present.
- For example, a student did a chemical analysis on a sample of salt X. His results showed the presence of sodium ions and bromide ions. His conclusion was the salt must be sodium bromide.
- The procedure for testing salts in the laboratory consists of the following general steps.
- Make initial observations of the physical properties of the salt.
- Study the action of heat on the salt.
- Make aqueous solution of the salt to test for anions and cations present.
- Carry out confirmatory tests for cations and anions.
People also ask
- Classification of Salts
- General Properties of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Action of Heat on Salts
- Test for Cations and Anions in Aqueous Solutions
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
Examining the colour and solubility of salts in water
- Preliminary examination on the physical properties of a salt such as colour and solubility in water can help to indicate that certain cations and anions might be present.
- Observations of physical properties only allow you to make inferences on the possibility of the presence of certain ions in the salts. This is because it is possible to have more than one salt sharing the same physical properties. For example:
(a) Iron(II) ions, nickel(II) ions and chromium(III) ions dissolve in water to produce green solutions.
(b) Sodium chloride and potassium carbonate are white solids. Both solids dissolve in water to produce colourless solutions. - Hence, observations of physical properties of salts cannot be used to confirm the identities of the ions. Its main purpose is to help us narrow down the choices of cations and anions present in a salt. Chemical tests still have to be carried out to confirm these ions.
Table lists the colours of some common salts.
| Salt | Colour | |
| Solid | Aqueous solution | |
| Potassium salts Sodium salts Ammonium salts Aluminium salts Calcium salts Lead(ll) salts Zinc salts (with colourless anions) | White | Colourless |
| Carbonate salts Chloride salts Nitrate salts Sulphate salts (with colourless cations) | White | Colourless |
| Iron(II) salts: Iron(II) chloride Iron(II) nitrate iron(II) sulphate | Green | Green |
| Iron(III) salts: Iron(III) chloride Iron(III) nitrate Iron(III) sulphate | Brown | Brown |
| Copper(II) salts: Copper(II) chloride Copper(II) nitrate Copper(II) sulphate | Blue | Blue |
| Copper(II) carbonate | Green | (Insoluble) |
Table shows the solubility of some common compounds.
| Compound | Solubility in water |
| Sodium, potassium and ammonium salts | All are soluble |
| Nitrate salts | All are soluble |
| Ethanoate salts | All are soluble |
| Chloride salts | All are soluble except AgCl, HgCl and PbCl2 |
| Sulphate salts | All are soluble except BaSO4, PbSO4 and CaSO4 |
| Carbonate salts | All are insoluble except Na2CO3, K2CO3 and (NH4)2CO3 |
| Metal oxides | All are insoluble except Na2O, K2O and CaO (slightly soluble) |
| Metal hydroxides | All are insoluble except NaOH, KOH and Ba(OH)2 |
Example: Preliminary examination of solid X gave the following observations. Identify solid X. Explain your answer.
- Green solid that insoluble in water
- Dissolved in dilute acid with effervescence to form a blue solution
Solution:
- The colour of an aqueous solution of a substance gives a more accurate inference about the nature of the ion present. Blue solution indicates presence of Cu2+ ion.
- When a metal reacts with an acid, effervescence due to hydrogen gas liberated is observed. Effervescence due to carbon dioxide gas is seen when an acid reacts with a carbonate salt. Most metals are grey solids. Hence, the green solid X has to be a carbonate salt. X is copper(II) carbonate.
Test for gases
- Gases are often produced from reactions carried out during laboratory tests on salts. Gases can be evolved when
(a) salts are heated.
(b) salts are reacted with acids or alkalis. - For example:
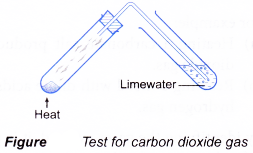
(a) Heating a carbonate salt produces carbon dioxide gas.
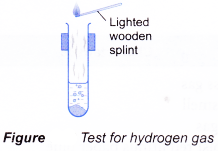
(b) Reacting a metal with dilute acids produces hydrogen gas. - By identifying the gases evolved, it is possible to infer the types of cations or anions present in a salt.
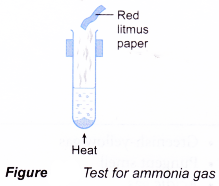
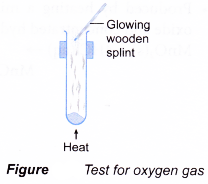
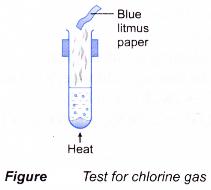
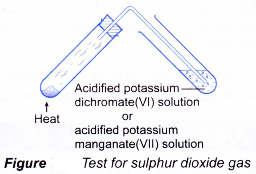
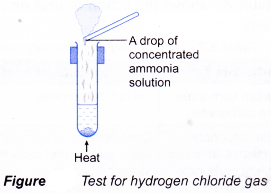
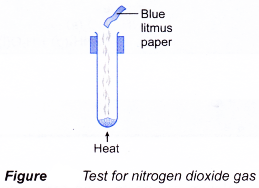
- A gas can be identified by its colour, smell, effect on litmus paper and reactions with special reagents.
| Gas | Characteristic |
| Ammonia Method:
Observation:
|
|
| Oxygen Method:
Observation:
|
|
| Carbon dioxide Method:
Observation:
|
|
| Hydrogen Method:
Observation:
|
|
| Chlorine Method:
Observation:
|
|
| Sulphur dioxide Method:
Observation:
|
|
| Hydrogen chloride Method:
Observation:
|
|
| Nitrogen dioxide Method:
Observation:
|
|