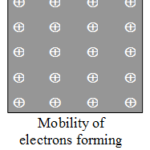
Explain Classification of Elements Elements An element is a substance which cannot be split up into two or more simpler substances by the usual chemical methods of applying heat, light or electric energy. An element cannot be split up into two (or more) simpler substances because it is made of only one kind of atoms. Ex. Hydrogen is an element because it cannot be … [Read more...] about Explain Classification of Elements
Elements
What are the types of Pure substances and Mixtures
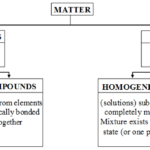
What are the types of Pure substances and Mixtures All the matter around us is not pure. The matter around us is of two types. In the previous chapter, we have learnt about the three states of matter. Before, understanding the chemical nature of matter, let us first understand the scientific meaning of the term chemical substance. The scientific meaning of the term chemical … [Read more...] about What are the types of Pure substances and Mixtures
What is the Need for Classification of Elements?
What is the Need for Classification of Elements? Need of Classification : It is difficult to study each and every element individually and to know its properties and uses. Therefore, they have been classified into groups on the basis of their similarities in properties. Basis of Classification : Classification is done on the basis of similarities in properties so that the … [Read more...] about What is the Need for Classification of Elements?
Modern Periodic Table and Its Significance
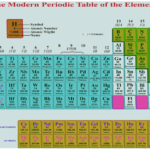
Modern Periodic Table and Its Significance Modern Periodic Table : Henry Moseley, an English physicist found that the atomic number (Z) was the fundamental property of an elements and not the atomic mass for classification of elements. Modern Periodic Law : ‘‘Properties of elements are periodic functions of their atomic numbers, i.e., the number of protons or electrons … [Read more...] about Modern Periodic Table and Its Significance