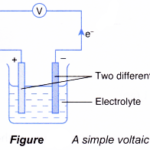
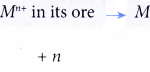Oxidation and Reduction in Chemical Cells In a simple voltaic cell, two different metals are in contact with an electrolyte.The more electropositive metal will become the negative terminal while the less electropositive metal will become the positive terminal.The chemical change that takes place at each electrode is actually a half-reaction … [Read more...] about Oxidation and Reduction in Chemical Cells
Electrolytic and Chemical Cells
Changing of iron(II) ions to iron(III) ions and vice versa
Changing of iron(II) ions to iron(III) ions and vice versaIron exhibits two oxidation numbers (a) +2 as iron(II) ion, Fe2+ (b) +3 as iron(III) ion, Fe3+ An aqueous solution containing iron(II) ions, Fe2+ is pale green in colour, whereas that containing iron(III) ions, Fe3+ is yellow/yellowish-brown/ brown in colour. Changing iron(II) ions to iron(III) ions is an … [Read more...] about Changing of iron(II) ions to iron(III) ions and vice versa
Oxidation and Reduction in Electrolytic Cells
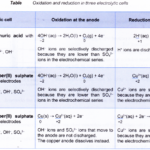
Oxidation and Reduction in Electrolytic Cells In an electrolytic cell, an electric current is passed through an electrolyte using electrodes. The electrolyte may be a molten ionic compound or an aqueous solution containing ions. The electrodes are usually inert conductors such as platinum or carbon. Sometimes active electrodes such as copper are used. … [Read more...] about Oxidation and Reduction in Electrolytic Cells
Electrolytic and Chemical Cells
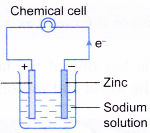
Electrolytic and Chemical CellsRedox reaction occurs in both electrolytic and chemical cells. An electrolytic cell is a device that uses electricity from an external source to drive a redox reaction. On the other hand, a chemical cell is a device that uses redox reaction to produce electricity. The two cells differ in the following … [Read more...] about Electrolytic and Chemical Cells
Application of the reactivity series of metals in the extraction of metals
Application of the reactivity series of metals in the extraction of metals Application of the reactivity series of metals towards oxygen in the extraction of metals:Only a few metals such as platinum, gold and silver are found free in nature. Other metals have to be extracted from their ores. Ores are naturally occurring rocks that contain high concentration … [Read more...] about Application of the reactivity series of metals in the extraction of metals