What is the monomer of natural rubber?
- The monomer unit for natural rubber is isoprene.
Its IUPAC name is 2-methylbut-1,3-diene.
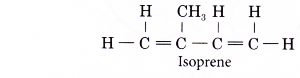
- An addition polymerisation joins thousands of isoprene units together to form poly(isoprene) or natural rubber.
Natural rubber properties
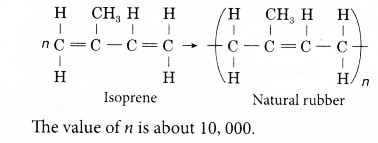
- Natural rubber is elastic.
(a) In its ordinary state, the rubber polymer chain is folded into a tangled mass.
(b) When it is stretched, the chain straightens out. It reverts back to its tangled state once the stretching force is released.
- Natural rubber is insoluble in water.
(a) The long hydrocarbon chains of natural rubber cannot dissolve in water.
(b) Natural rubber is soluble in organic solvents such as benzene, petrol, carbon disulphide and chlorohydrocarbons. - Natural rubber is unstable to heat.
(a) When it is warmed above 50°C, it softens and becomes sticky. Heating it to temperatures above 200°C will cause it to decompose.
(b) When natural rubber is cooled, it becomes hard and brittle. It behaves like plastic. - Natural rubber is unstable to oxidation.
(a) The presence of double bonds in the polymer chain makes it susceptible to oxidation.
(b) Atmospheric oxidation occurs when oxygen and ozone from the air together with ultraviolet radiation breaks up the polymer chains.
How vulcanized rubber is made?
Vulcanisation of rubber:
- Vulcanisation is a manufacturing process discovered by Charles Goodyear in 1839 to convert raw rubber into a tough useful product.
- In this process, about 1 – 3% by weight of sulphur is added to raw rubber and the mixture is heated carefully.
- Sulphur atoms form cross-links between adjacent chains of rubber polymer at the carbon-carbon double bonds.
- The number of sulphur atoms in the cross-links is usually one to four.
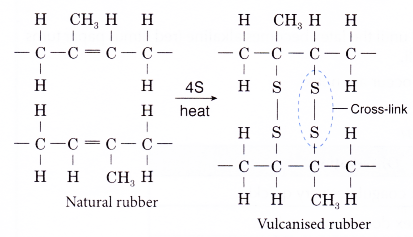
- The cross-linking improves the properties of raw rubber, making vulcanised rubber.
(a) a tougher material that is more resistant to oxidation.
(b) more elastic as the cross-linked chains can revert back to their original positions.
(c) more heat resistant which means the vulcanised rubber is less soft and sticky on warming.
(d) less soluble in organic solvent.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
What can natural rubber be used for?
Uses of natural rubber:
- Uses of natural rubber are limited to making
(a) mixtures of latex cement and rubberised bitumen for tarring roads
(b) insulators for electrical appliances and cables
(c) friction enhancers such as shoe soles and door stoppers
(d) rubber hoses, rubber caps and rubber bands
(e) gloves - Natural rubber is processed to become vulcanised rubber which has better properties – more elastic and more stable to heat and oxidation.
- Vulcanised rubber is used to make tyres, gloves and tubing.
- Carbon is added to vulcanised rubber in tyres to make them tougher while maintaining their elasticity.
- Many buildings, particularly those that are near to railways or situated in earthquake prone areas, are now built on rubber blocks or rubber bearings which can help to absorb vibration.
Will vulcanisation increase the elasticity of rubber experiment
Aim: To investigate the effect of vulcanisation on the elasticity of rubber.
Problem statement: Will vulcanisation increase the elasticity of rubber?
Hypothesis: When rubber is vulcanised, it will increase its elasticity.
Operational definition:
Elasticity is the ability of the rubber strip to be stretched by hanging weights of different masses to it and return to its original length when the weights have been removed. The greater the mass of the weight used, the more elastic the rubber.
Variables:
(a) Manipulated variable : Types of rubber
(b) Responding variable : Length of rubber strip
(c) Controlled variable : Size of rubber strip, mass of weights
Materials: Latex, solution of dichloride disulphide in methylbenzene.
Procedure:
- Two strips of natural rubber of the same dimension (100 mm x 10 mm x 1 mm) are prepared from the latex provided.
- One strip of rubber is vulcanised by dipping it into dichloride disulphide solution for about 5 minutes. The vulcanised rubber strip is then removed and dried in air.
- The vulcanised and unvulcanised rubber strips are hung using bulldog clips as shown in Figure.
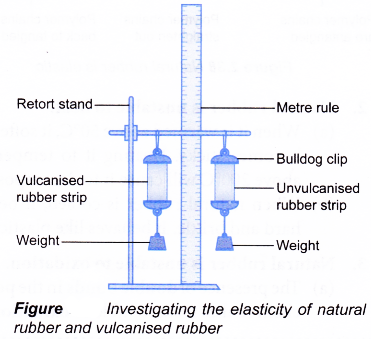
- The lengths of the two strips are measured.
- 50-gram weight is hung to each of the two strips. The lengths of the two strips are measured.
- The weights are removed and the lengths of the two strips are measured again.
- Repeat steps 4 to 6 using weights in 50-gram increments until each of the strips does not return to its original length.
- Record and tabulate your results.
Observations:
| Mass of weight added (g) | Length of natural rubber strip (cm) | Length of vulcanised rubber strip (cm) | ||
| Before adding weight | After removing weight | Before adding weight | After removing weight | |
| 50 | 100 | 100 | 100 | 100 |
| 100 | 100 | 100 | 100 | 100 |
| 150 | 100 | 120 | 100 | 100 |
| 200 | – | – | 100 | 100 |
| 250 | – | – | 100 | 110 |
Discussion:
- Unvulcanised rubber does not return to its original length after a 150-gram weight is used.
- Vulcanised rubber does not return to its original length after a 250-gram weight is used.
- Hence, vulcanised rubber is more elastic because it can return to its original state when it is subjected to a stronger force.
Conclusion:
The hypothesis can be accepted.