Concept of the pH Scale
The pH Scale :
A litmus solution or litmus paper can be used to determine whether a given solution is acidic or basic. But suppose you have two acidic solutions containing different amounts of acids in them. How can you say which solution is more acidic? Similar is the case with the basic solutions. This problem is solved by using a scale known as the pH scale introduced by S P Sorensen in 1909. pH of a solution indicates which solution is more acidic or more basic than the other.
The acidity or basicity (alkalinity) of a solution is usually expressed in terms of a function of the H+ ion concentration. This function is called the pH of a solution.
The pH of an aqueous solution is the negative logarithm of its H+ ion concentration. That is,
pH = –log [H+].
pOH = –log [OH–].
Note: [H+] and [OH–] denote the concentrations of H+ and OH– ions respectively.
The concentrations of H+ and OH– ions in aqueous solutions are usually very small numbers and therefore difficult to work with. Since pH is the negative logarithm of [H+], we get positive’ numbers and the inconvenience of dealing with small numbers is eliminated.
It should be noted here that pH is only a number, because we can take the logarithm of a number and not of a unit. Therefore, pH of a solution is a dimensionless quantity.
In a neutral solution, [H+] = 1.0 x 10–7 M.
∴ pH = –log (1.0 × 10–7) = 7.
We can say that the pH of a neutral solution is 7. In an acidic solution, [H+] > 1.0 × 10–7 M.
Let us assume, [H+] = 1.0 × 10–5 M.
∴ pH = –log (1.0 × 10–5) = 5.
Here, we find that the pH of an acidic solution is less than 7.
In an alkaline solution, [H+] < 1.0 × 10–7 M. Let
as assume, [H+] = 1.0 × 10–9 M.
∴ pH = –log (1.0 × 10–9) = 9.
In other words, the pH of an alkaline solution is more than 7.
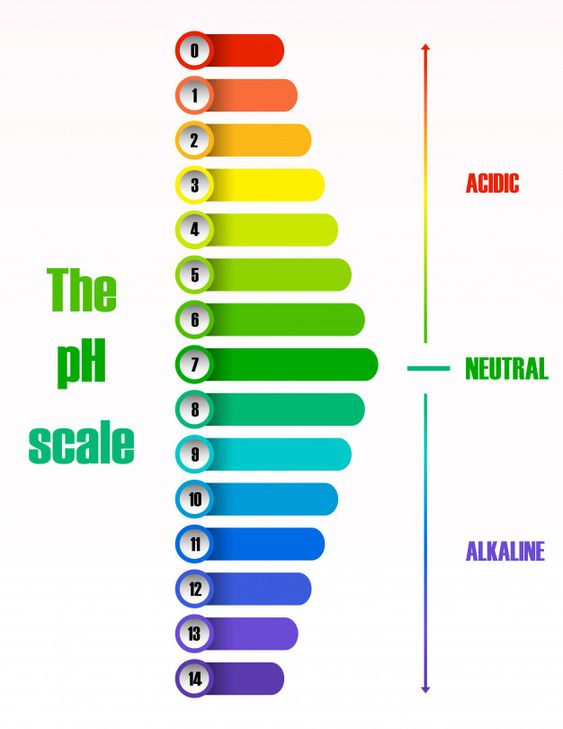
The pH of different solutions at 298 K can now be expressed on the pH scale as shown below.
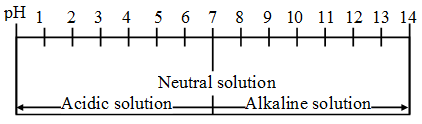
Rules for pH scale (at 298 K)
- Acidic solutions have pH less than 7.
- The lower the pH, the more acidic is the solution.
- Neutral solutions or pure water has pH equal to 7.
- Basic solutions have pH greater than 7.
- The higher the pH, the more basic is the solution.
The pH values of some common solutions
Substance | pH |
Gastric juice | 1.0 |
| Lemon juice | 2.5 |
Vinegar | 3.0 |
Wine | 3.5 |
| Tomato juice | 4.1 |
Acid rain | 5.6 |
| Urine | 6.0 |
Milk | 6.5 |
Pure water | 7 |
| Blood | 7.4 |
| Lime water | 11.0 |
How is pH measured?
The pH of a solution is generally determined with the help of a pH paper, or universal indicator. The pH paper gives a particular colour with a solution of particular pH. The colour is compared with a chart which has different colours at different pH values.
People also ask
- Role of pH in everyday life
- What is the pH of a salt solution
- Relationship between pH values and molarity of acids and alkalis
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?